Biostime® Children Probiotics With 2’-FL HMO#
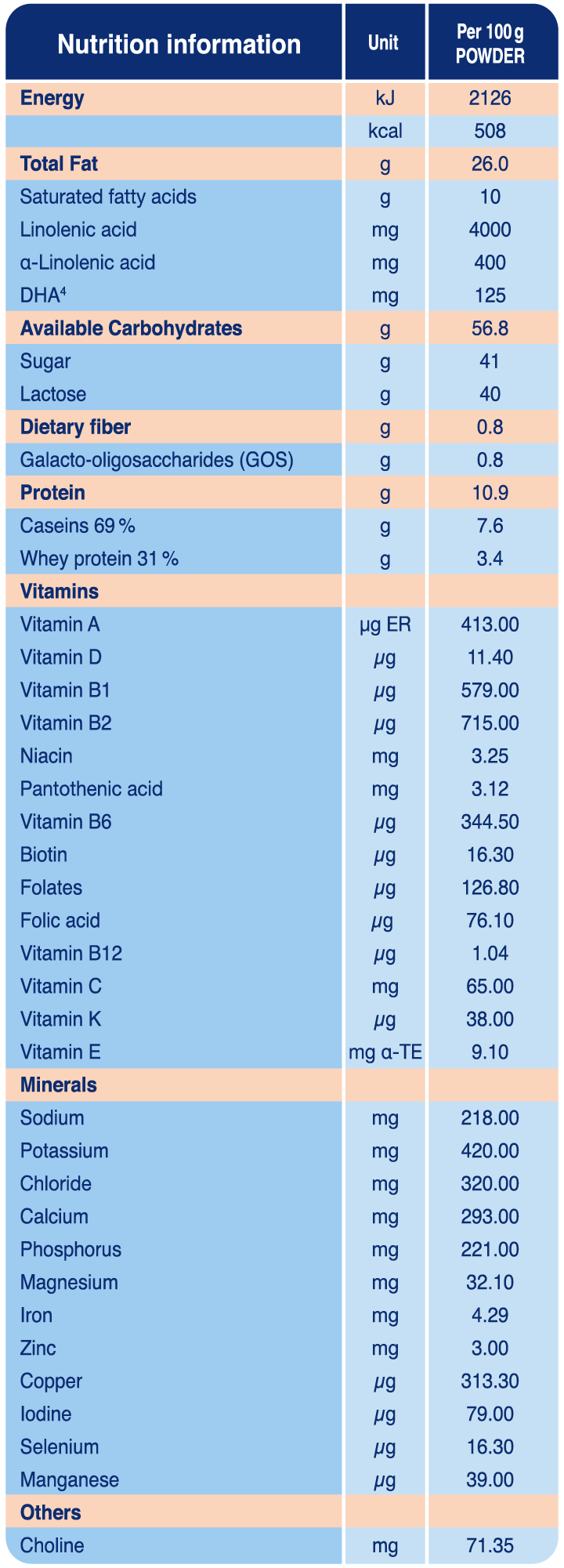
Biostime® SN-2 BIO PLUS Premium Organic Follow-Up Formula provides comprehensive nutrition to support the needs of growing babies from 6-12 months who are not being breast-fed or are mixed feeding. Made from certified organic ingredients and with the inclusion of a prebiotic and probiotic, Biostime® SN-2 BIO PLUS Premium Organic Follow-Up Formula is formulated to help support the development of a healthy microbiome.1



Are you a Healthcare Professional?
Important Notice and Declaration
Breast milk is best for babies. Professional advice should be followed before using an infant formula. Introducing partial bottle feeding could negatively affect breast feeding. Good maternal nutrition is important for breast feeding and reversing a decision not to breast feed may be difficult. Infant formula should always be used as directed. Proper use of an infant formula is important to the health of the infant. Social and financial implications should be considered when selecting a method of feeding.
The information provided on this website is intended for use by healthcare professionals only. It is a condition of use of this site that you are a healthcare professional within the meaning of regulations within your country of practice. Then denoting below Yes I am/No icons. For Healthcare Professionals based in Australia : It is a condition of use of this site that you are a healthcare professional within the meaning of the Marketing in Australia of Infant Formulas (MAIF) Agreement or the Therapeutic Goods Act.